|
Workshop XV
Will be held - Virtually
Dates - November 19 and 20
2025 Times are EST
- Chat
- Day 1 Afternoon
- Day 2 Morning
Workshop XIV
Thanks to everyone who attended the workshop in person and online in Washington D.C. this past October 17-19 2023
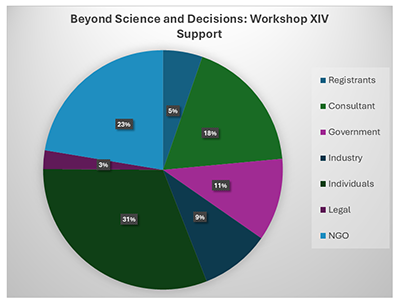
Click on the graphic to enlarge
Workshop 14 Highlights
 Download the 29-page Highlights of Workshop XIV document Download the 29-page Highlights of Workshop XIV document 
for a suggested donation of $150.
“If a donation is not possible, please contact Michael Dourson at dourson@tera.org.”
Presentations
To obtain a PDF of a presentation Click Here
Use your mouse or touch pad to maneuver the bookshelf up and down. Click on the presentation you want. It will open in a new window or tab.
Case Studies:
Case Study Summary: Differences Among Perfluorooctanoate (PFOA) Safe Doses
Workshop XIII
Date: February 2022
Location: Washington D.C.
Workshop XlIl Case Presentations
Workshop XII
Time: Held on February 24 and 25 of 2021
Location: Virtual Meeting
Workshop XlI Case Presentations
ARA XII Presentations
210225-ARA Metals Toolbox Schlekat:
ARA Presentation RDanzeisen VViegas version 20210221:
Gadagbui ARA PFOA :
Holistic risk assessment:
TCEQ Presentation:
Berezow---Junk risk assessment:
Clewell TERA PFOA presentation:
OARS WEEL:
Workshop XlI Case Studies
Research Case Study 1: Instantaneous Comparison Values (ICVs) & Acute Action Levels (AALs) for Use During In-Motion Monitoring & Emergency Events
Research Case Summary 1: Comparing Human Observational Studies with Clinical Findings: The Half-life of Perfluorooctanoate (PFOA)
Research Case Summary 2: A tiered approach to the assessment of inhaled cobalt compounds Version: 1.1
Workshop XII References
Reference 1: Campbell 2016 SOT PFOA Poster
Reference 2: Conventino et al, 2018
Reference 3: DeSiilva et al, 2020 PFAS Exposure
Reference 4: Dourson et al, 2019
Reference 5: Elcombe et al, 2013
Reference 6: Mikkonen et al, 2020
Reference 7: Russell et al, 2015
Workshop XI
Time: Held on February 18, 19, and 20, 2020
Location: Cincinnati, Ohio
Workshop XI Case Studies
Research Case Study 1: Occupational Exposure Banding 2.0: Characterizing Risks for Chemicals with Limited Data
Research Case Study 2: Use And Application Of Real-Time Exposure Monitoring
Research Case Study 3: Applying Hypothesis-Testing Methods to Help Inform Causality Conclusions from Epidemiology Studies
Research Case Study 4: Understanding Weight-of-Evidence of Ototoxicity from Co-Exposures to Noise and Chemicals in the Workplace
a.
Use of the kurtosis statistic in an evaluation of the effects of noise and solvent
exposures on the hearing thresholds of workers: An exploratory study
b.
Chemical exposure and hearing loss
c. Lentz 2015
Research Case Study 5: Risk/Benefit Methods for Carcinogenicity/Sterilization with Ethylene Oxide as an Example
a. Attachment 1
b. Attachment 2
Research Case Study 6: Risk assessment methods of flavoring in e-vapor products
Workshop XI Presentations
Feb 18
1. Taking stock of the field
2. Occupational Risk Assessment 2020 . . .and Beyond
3. Occupational Exposure Banding 2.0: A Preliminary Case Study
4. Use and Application of Real-Time Exposure Monitoring
Feb 19
1. Applying Hypothesis-Testing Methods to Help Inform Causality Conclusions from Epidemiology Studies
2. Data Derived Extrapolation Factors (DDEFs) for Developmental Toxicity: A Research Case Study With Perfluorooctanoate (PFOA)
3. A Repair for Non-Cancer Assessment: Introducing the Truly Adverse Dose (the TAD)
4. A Call for Establishing an Interagency Epidemiology Peer Review Council (IEPRC) for Chemicals
5. Novel and Integrated Approaches to modelling aggregate exposures to chemicals across different conditions of use and routes of exposure
6.
Using the Matrix to bridge the gap between epidemiology and risk assessment
7.
Understanding Weight-of-Evidence from Co-Exposures to Noise and Chemicals in the Workplace A Preliminary Assessment
Feb 20
1. Risk-Risk Tradeoff Methods: Carcinogenicity/Sterilization with Ethylene Oxide (EtO) as an Example
2. Risk Assessment Strategy of Flavor Ingredients in e-Vapor Products
Workshop X
February 26 & 27, 2019
Workshop IX
June 9-10, 2015 University of Cincinnati
Workshop VIII
May 21-22, 2014, Austin, TX,
Webinar
Workshop VII
November 13, 2013 12-3PM EST
Webinar
Overview
Workshop VII was held November 13, 2013 via webinar. The workshop was open to the public. The workshop focused on enhancements made to the Dose Response Framework, and obtaining feedback and ideas from the panel and participants on further needed changes.
 Workshop Series General Information Workshop Series General Information
 Workshop VI Workshop VI - May 28-29, 2013, Arlington, VA, U.S. Environmental Protection Agency
May 28, 29, 30, 2013
Potomac Yard - Arlington, VA
In person & web participation available
 Workshop V - November 2, 2012 Webinar Workshop V - November 2, 2012 Webinar
 Workshop IV - May 22-24, 2012,
Austin, Texas, Texas Commission on Environmental Quality Workshop IV - May 22-24, 2012,
Austin, Texas, Texas Commission on Environmental Quality
 Workshop III - May 4, 5, & 6,
2011 Workshop III - May 4, 5, & 6,
2011, Noblis; 3150 Fairview Park Drive South, Falls Church,
Virginia
 Workshop II - October 11-13, 2010 Workshop II - October 11-13, 2010, Crystal City, Virginia (In
tandem with the Federal & State Risk Assessment & Toxicology
Committee)
 Workshop I - March 16-18, 2010, Texas Commission on Environmental Quality; Austin, Texas Workshop I - March 16-18, 2010, Texas Commission on Environmental Quality; Austin, Texas
Background & Purpose:
The workshop series is continuing and expanding upon the discussion set forth by Science and Decisions: Advancement of Risk Assessment (NAS, 2009); these meetings are conducted under the aegis of the Alliance for Risk Assessment (ARA), a broad-based non-profit, government and NGO coalition. The first phase of the workshop series was three workshops over the course of about a year. The first workshop focused on brainstorming and selection of case studies illustrating various dose-response methods for different problem formulations. A broad range of case studies proposed at the first workshop was then developed by workshop participants and discussed by the Science Panel at the second workshop. In considering the case studies, the Science Panel members provided input on the utility of the case study methods to address specific problem formulations, and identified areas for additional development. The Science Panel and interested workshop participants developed an interactive framework for organizing case study methods, and the Panel used the framework to identify additional case studies that address important gaps in methodology; the third workshop focused on these case studies and associated issues. The framework references specific risk assessment methods, illustrated by case studies, and is intended for use by risk assessors and managers in a variety of settings (e.g., federal, state, and local agencies, industry). It is based on the fundamental premise that the appropriate methodology for dose-response assessment is necessarily based on objectives specific to that application, including varying levels of analysis. A manuscript describing the framework and workshop process is in preparation.
The workshop series is transitioning to an “evergreen” approach, including a standing panel that reviews methods and issues on a semi-annual basis, leading to updating of the framework.
General Workshop Objectives:
- Additionally develop the content of the NAS (2009) report on improving the risk assessment process to develop a compendium of practical, problem-driven approaches for “fit for purpose” risk assessments, linking methods with specific problem formulations (e.g., prioritization, screening, and in-depth assessment) for use by risk managers at a variety of levels (e.g., states, regional managers, people in a variety of agencies, and in the private sector).
- Implement a multi-stakeholder approach to share information, ideas and techniques in support of developing practical problem-driven risk assessment methods compendium.
Specific Workshop Objectives:
- Identify useful dose-response techniques for specific issues, including consideration of relevant data, characterization of assumptions, strengths and limitations, and how the techniques address key considerations in the dose-response.
- These techniques should appropriately reflect the relevant biology (including the biology of thresholds), and mode of action information, at a level of detail appropriate for the identified issue.
- Provide methods to explicitly address human variability in cancer assessment, and enhance the consideration of human variability in noncancer assessment, including explicit consideration of underlying disease processes, as appropriate for the relevant risk assessment context.
- Identify methods for calculating the probability of response for noncancer endpoints, as appropriate for the relevant risk assessment context.
- Develop a risk methods compendium that will serve as a resource for regulators and scientists on key considerations for applying selected dose-response techniques for various problem formulations, with suggested techniques and resources.
Roles
Steering Committee
The Alliance for Risk Assessment Steering Committee (ARA SC) provides oversight of the workshop series. The Steering Committee advises the Risk Assessment Advisory Committee (RAAC) on charge questions and has the final decision on members of the Science Panel after a review of all nominations. The ARA SC membership has included of a broad range of state, tribal, federal government, academic, and environmental NGO representatives. See ARA Steering Committee.
Science Panel
The Panel provides input on case study methods being proposed to enhance the risk framework. Panel members provide input on the utility of the case study methods to address specific problem formulations, and identify areas for additional development of the case study and/or method. Inclusion of a method or case study in the framework as an illustration of a useful technique does not imply panel acceptance of the chemical-specific outcome. Core panel members will serve for 2-3 years; members may be added to the standing panel to ensure expertise on specific topics. Panel members are selected from a diversity of affiliations and areas of expertise, particularly biology/toxicology, risk assessment, and statistical/modeling. See Science Panel
Risk Assessment Advisory Committee (RAAC)
The workshop sponsors include state, federal, industry, and NGO representatives. The Risk Assessment Advisory Committee (RAAC) represents the various sponsors and has final decision in the development of workshop structure and charge questions, and the recruitment of presenters, after consultation with the ARA Steering Committee and Science Panel. See Risk Assessment Advisory Committee.
Link to sponsor organizations
Funding
The "Beyond Science and Decisions: From Problem Formulation to Dose Response" Alliance for Risk Assessment (ARA) project is funded through donations of money for travel, meeting expenses and contractor time, and by donated time. The distribution of donated money for the first 3 meetings in this series was 53%, 32%, and 14%, for industry, government and NGO groups, respectively; the distribution of donated time was roughly 33% for each sector. The distributions of time and money were different because many government and NGO groups were able to donate time, but not money.
The continuation of this project through the development of a standing science panel in meetings 4 and onward is envisioned to be based on donated money and time, as before. These resources are anticipated to come from different organizations that desire technical feedback on case studies that might fit within the developing framework, and from small grants to continue the development of the framework. In addition, this funding is anticipated to cover a limited number of case studies and/or methods papers on broader topics chosen by the science panel. In 2012, the distribution of grant money is anticipated to be 40%, 48%, and 13%, for industry, government and NGO groups, respectively. The distribution of donated time is anticipated to be similar across sectors.
Beyond Science & Decisions In the News
Ginsberg et al. The NRC Silver Book: The Case for Improving Non-Cancer Risk Assessment. Guest Perspective. Risk Policy Report 17 (37) Sept. 14, 2010 link
Hegstad M. Two Years On, Assessors Urge NAS to Clarify Advice on Linear Risk Method. Guest Perspective. Risk Policy Report 17(43). October 26, 2010. link
Hegstad M. Alliance Plans Panel To Address Key Scientific Issues In Risk Assessment. Risk Policy Report
18, (29). July 19, 2011 link
Hegstad M. EPA Framework Emphasizes Risk Management Options In Assessments Risk Policy Report 18(22). May 31, 2011. link
Meek B, Dourson M. Integrating Cancer and Non-Cancer Dose Response Assessment Approaches to Risk Assessment: The Role of Mode of Action. Risk Policy Report 17(39) September, 28, 2010 link
RASS August 2011
RSESS Winter 2011 link
RSESS Winter 2012 link
References
International Programme for Chemical Safety
(2005) IPCS Framework for Analyzing the Relevance of a Cancer Mode of Action
for Humans.
http://www.who.int/ipcs/methods/harmonization/areas/cancer/en/index.html
National Academy of Sciences (2009). Science
and Decisions: Advancing Risk Assessment (NAS Final Report).
http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=202175
United States (2005). Guidelines for
Carcinogen Risk Assessment.
Scientific Society Presentations
Society for Risk Analysis 2010 Annual Meeting Presentations from the symposium on “Beyond Science and Decisions
2011 Society for Risk Analysis Symposium: Improving Problem
Formulation and Dose-Response Beyond Science
and Decisions
Sponsored by: Dose-Response Specialty Group
Co-Chairs: Julie Fitzpatrick,
Rick Becker
Tox Forum 2012
Society of Toxicology 2012
- Borgert C., Sargent E., Casella G., Dietrich D., McCarty., L, Golden, R., The Human Relevant Potency Threshold: Reducing Uncertainty by Human Calibration of Cumulative Risk Assessments.***SOT RASS Top 10 published paper for Demonstrating an Application of Risk Assessment***
- Gentry, R., Van Landingham, C., Aylward, L., Hays, S. Use of Biomarkers in the Benchmark Dose Method
- Haber, L. Beyond Science and Decisions: From Problem Formulation to Dose-Response - Framework
- Kroner, O. Haber, L. Where the Rubber Meets the Road: A Practical Methods Compendium for Risk Assessors
- Pottenger L.H; J. A. Swenberg; J. S. Bus, Endogenous DNA Damage: Considerations for Dose-Response and Risk Assessment **SOT RASS Top 10 abstracts***
- Price, P., Juberg, D., Bartels, M., PBPK/PD Modeling of Key Events in a Toxicity Pathway - Implications for Determining Population Thresholds ***SOT RASS Top 10 abstracts***
- Thompson, R., Haws, L., Harris M., Gatto, N., Proctor, D. Application of the U.S. EPA Mode of Action Framework for Purposes of Guiding Future Research: A Case Study Involving the Oral Carcinogenicity of Hexavalent Chromium. ***SOT RASS Top 10 published paper for Advancing the Science of Risk Assessment***
Society for Risk Analysis - New England Chapter
Presentation at Society for Risk Analysis 2012
*Fit for purpose: Consistent with the NRC (2009) report, this workshop defines “fit for purpose” as recognizing that the nature and extent of the assessment needs to be considered in the problem formulation stage, with level and complexity to be no greater than that needed to identify the best choice among risk management options. In practice, the workshop recognizes that this is accomplished by having a variety of available tools (e.g., tools for acute vs. chronic exposures) and using tiered approaches, proceeding down the tiering only as far as necessary to set an issue, exposure or chemical aside (as not of concern) or to target it for further assessment and/or management.
Adapted from: Meek, M.E., M. Bolger, J.S. Bus, J. Christopher, R.B. Conolly, R.J. Lewis, G. Paoli, R. Schoeny, L.T. Haber, A.B. Rosenstein, M.L. Dourson. 2013. A Framework for Fit-for-Purpose Dose Response Assessment. Manuscript in review
For more information, please contact
Valerie Ayers.
|
|
ARA Workshop Series on Zenodo – Full citations with URLs
Workshop I
https://doi.org/10.5281/zenodo.12751642
Workshop II
https://doi.org/10.5281/zenodo.12751766
Workshop III
https://doi.org/10.5281/zenodo.12751798
Workshop IV
https://doi.org/10.5281/zenodo.12751827
Workshop V
https://doi.org/10.5281/zenodo.12760655
Workshop VI
https://doi.org/10.5281/zenodo.12761046
Workshop VII
https://doi.org/10.5281/zenodo.12810138
Workshop VIII
https://doi.org/10.5281/zenodo.12761095
Workshop IX
https://doi.org/10.5281/zenodo.12772768
Workshop X
https://doi.org/10.5281/zenodo.12772833
Workshop XI
https://doi.org/10.5281/zenodo.12772856
Workshop XII
https://doi.org/10.5281/zenodo.12772891
Workshop XIII
https://doi.org/10.5281/zenodo.12772954
Workshop Collaborators






















|

 Download the 29-page Highlights of Workshop XIV document
Download the 29-page Highlights of Workshop XIV document 
 Workshop Series General Information
Workshop Series General Information















